.png--diagram-flowchart-example.png)
Design elements - Aromatic hydrocarbons (arenes) | Phenols | Aromatics - Vector stencils library | Aromatic Hydrocarbon Reaction

Molecule Chemistry Aromatic hydrocarbon Benzene, aromatic ring, biology, chemistry, acid png | PNGWing

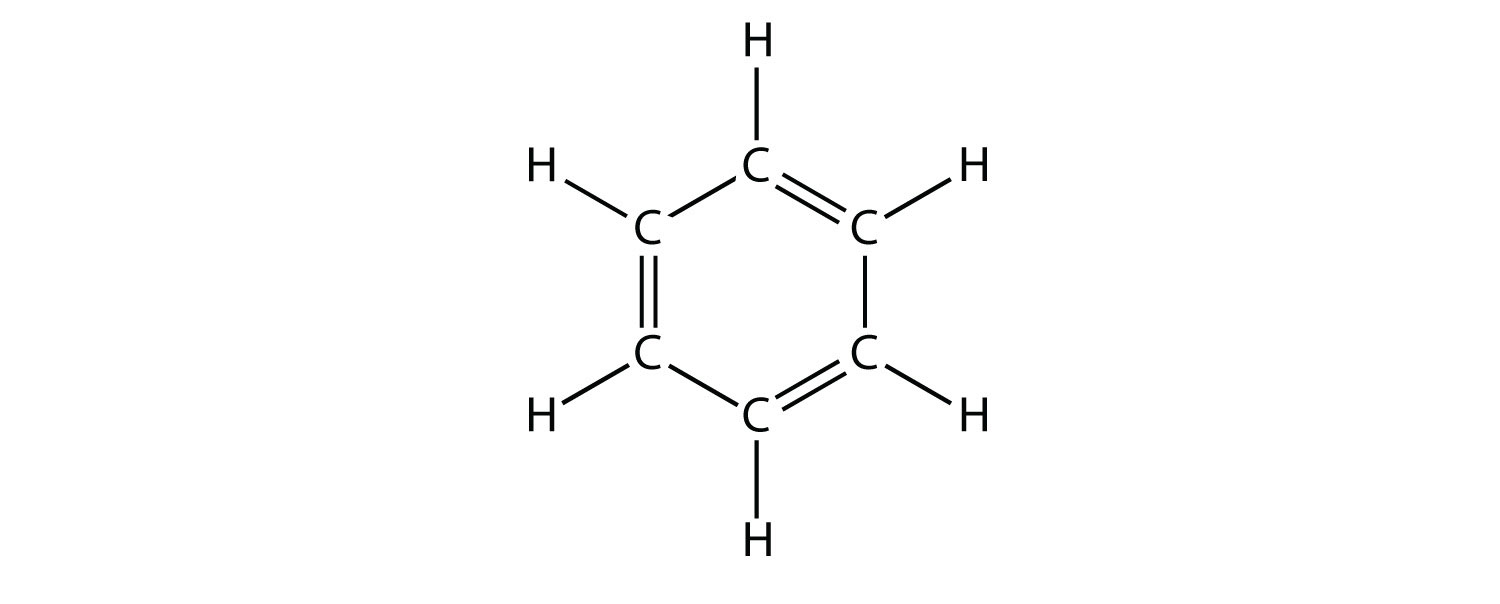
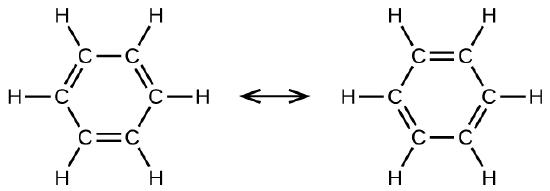
A hydrocarbon consists of a benzene ring and two methyl groups. The methyl groups are located at opposite carbons and the molecule is perfectly symmetrical. Which option correctly describes the numbers of
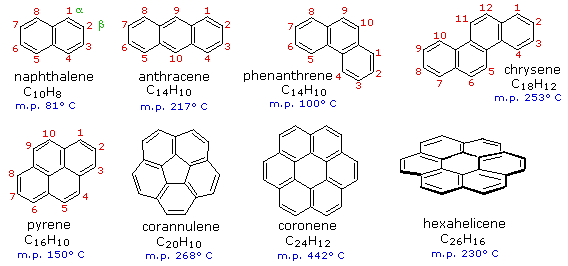
![Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10.[1] The molecule consists of a cyclopentane ring fused with 5 benzene ri Stock Photo - Alamy Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10.[1] The molecule consists of a cyclopentane ring fused with 5 benzene ri Stock Photo - Alamy](https://c8.alamy.com/comp/2BJHN82/corannulene-is-a-polycyclic-aromatic-hydrocarbon-with-chemical-formula-c20h10-1-the-molecule-consists-of-a-cyclopentane-ring-fused-with-5-benzene-ri-2BJHN82.jpg)
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10.[1] The molecule consists of a cyclopentane ring fused with 5 benzene ri Stock Photo - Alamy

Benzene Chemistry Aromatic hydrocarbon Friedel-Crafts-Acylation Deuterium, benzene ring, sphere, chemistry png | PNGEgg